
Below are examples of typical results seen in historical lots. Optimization of the assay conditions may be required to efficiently utilize these assays for the measurement of these analytes in animal plasmas. Charles River supports monoclonal antibody research with our extensive expertise in the initial stages of safety assessment for therapeutic large molecules. In their Article on the risk of COVID-19 in health-care workers in Denmark, Kasper Iversen and colleagues1 used a point-of-care test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG and IgM antibodies developed by Livzon Diagnostics (Zhuhai, Guangdong, China). Plasmas with reactivities of less than 1% are listed as negative. The values given are the re-activities relative to normal human reference plasma when the test plasmas are assayed under the conditions specified in the protocol for each assay. See product insert for lot specific testing results. Cross-reactivity between antigens occurs when an antibody raised against one specific antigen has a competing high affinity toward a different antigen, therefore the antibody is able to recognize a protein which is different to the one it was raised against. An antibody may demonstrate a greater spectrum of cross reactivity in applications such as immunoblotting and immunohistochemistry where antigen precipitation is not required. What is cross-reactivity An antibody has a specific amino acid sequence (the Fab region) that dictates its affinity for a specific antigen. A number of antibodies may react with fewer recognition sites on the antigen of another species and be unable to form an insoluble immune complex (this is observed with many monoclonal antibodies). In order to dissociate antigenantibody complexes formed by antibodies with an optimum reaction temperature of +37C, one would have to raise the temperature to about +56C. At this temperature, the speed of their reaction is also increased. The DID technique has limitations and a negative result should not necessarily be interpreted as non-recognition of an antigen. Most IgG antibodies react best with the corresponding antigens at +37C. The target analytes of lateral flow assays include infectious disease agents, drugs of abuse, hormones, cancer markers, food contaminants and agricultural contaminants 1, 2. The double immunodiffusion (DID) technique used tests the ability of an antibody to precipitate an antigen in an agarose gel. Lateral flow assays based on Immunochromatography principles exhibit a wide range of applications as diagnostic as well as prognostic tool.
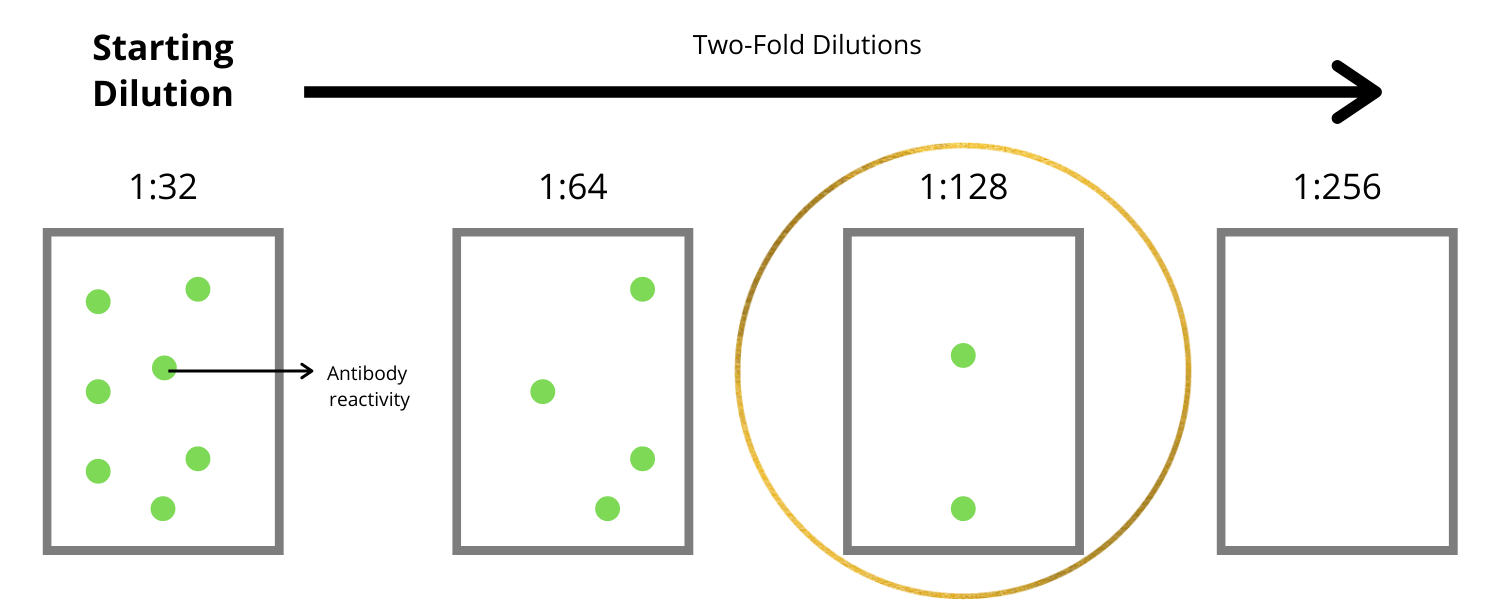
Each antibody listed has been tested for reactivity to the plasma of a variety of species.


 0 kommentar(er)
0 kommentar(er)
